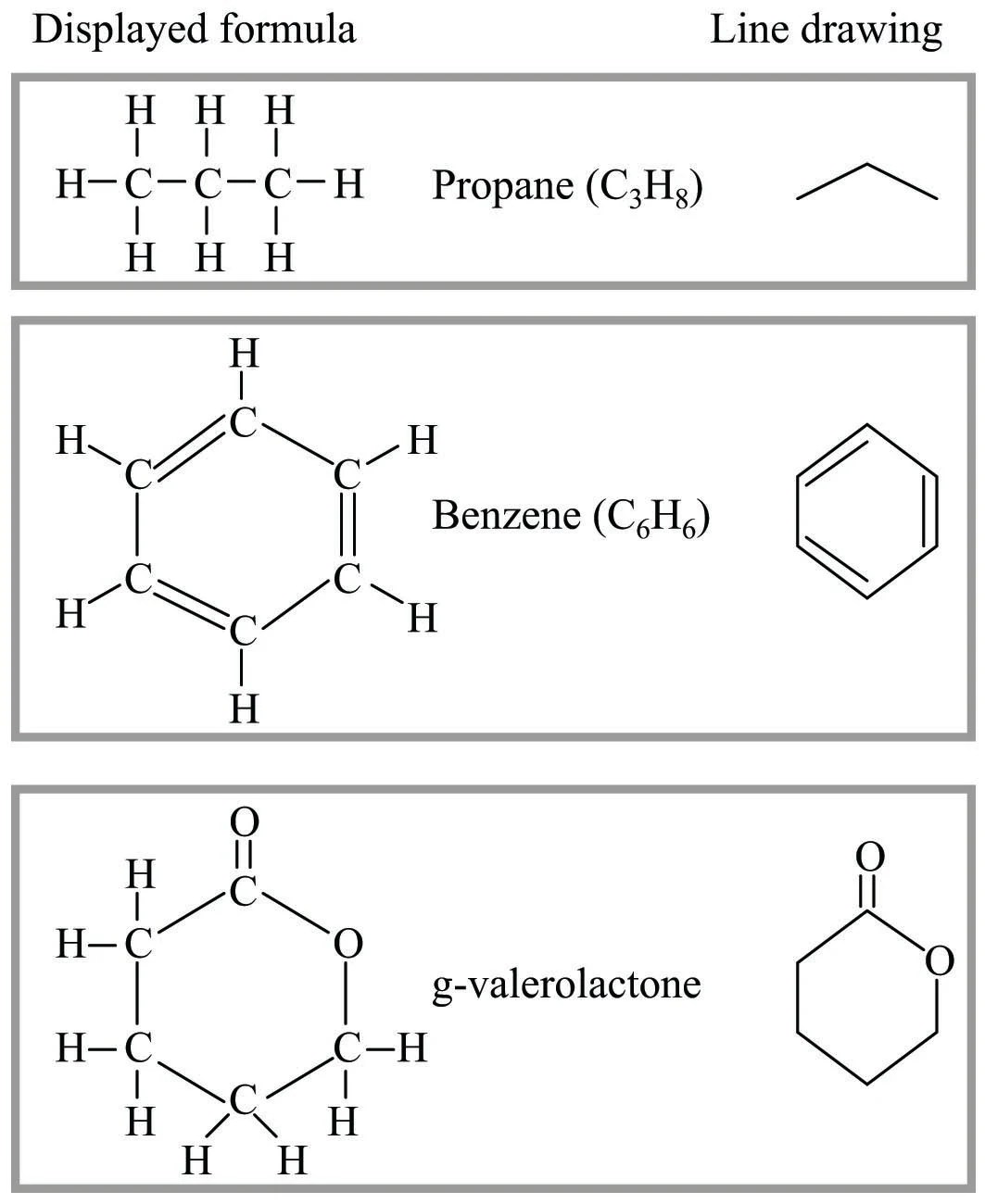
Seznamy 55 Atom Notation
Seznamy 55 Atom Notation. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would … The element symbol is used in addition to a subscript and superscript showing the atomic number and atomic weight. The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number When writing it, the 38 goes directly.
Nejchladnější Atomic Theory And Chemical Symbols Chemistry In Industrial Instrumentation Automation Textbook
For example, hydrogen has one electron in the … Check out the examples below for clarification. 27.10.2006 · the atom's atomic number is the number of protons of the atom—thus, it is also the number of electrons in an atom with 0 charge.Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words.
For phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript. When writing it, the 38 goes directly underneath the 88. 16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr. Video atomic notation demonstrated example 1: If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would …

For example, hydrogen has one electron in the … The element symbol is used in addition to a subscript and superscript showing the atomic number and atomic weight. When writing it, the 38 goes directly. Video atomic notation demonstrated example 1: For example, hydrogen has one electron in the … X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation. 27.10.2006 · the atom's atomic number is the number of protons of the atom—thus, it is also the number of electrons in an atom with 0 charge. When writing it, the 38 goes directly underneath the 88. For phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript. 16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr. Check out the examples below for clarification. Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words.

For atoms, the notation consists of a sequence of atomic subshell labels (e.g.. . 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right.

When writing it, the 38 goes directly.. Video atomic notation demonstrated example 1: 27.10.2006 · the atom's atomic number is the number of protons of the atom—thus, it is also the number of electrons in an atom with 0 charge. 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation. The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number The element symbol is used in addition to a subscript and superscript showing the atomic number and atomic weight. For phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript. When writing it, the 38 goes directly underneath the 88. For example, hydrogen has one electron in the … 16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr.. 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right.

For phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would … When writing it, the 38 goes directly. Atomic notation atomic notation is a method of representing information about an atom or ion. For atoms, the notation consists of a sequence of atomic subshell labels (e.g. For phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript. Check out the examples below for clarification. The element symbol is used in addition to a subscript and superscript showing the atomic number and atomic weight.

If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would … 27.10.2006 · the atom's atomic number is the number of protons of the atom—thus, it is also the number of electrons in an atom with 0 charge. For phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript. The element symbol is used in addition to a subscript and superscript showing the atomic number and atomic weight. When writing it, the 38 goes directly underneath the 88. For example, hydrogen has one electron in the … Video atomic notation demonstrated example 1: Check out the examples below for clarification... For atoms, the notation consists of a sequence of atomic subshell labels (e.g.

For phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript... X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation. Atomic notation atomic notation is a method of representing information about an atom or ion. 16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr. When writing it, the 38 goes directly. The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number For example, hydrogen has one electron in the … If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would … When writing it, the 38 goes directly underneath the 88. 27.10.2006 · the atom's atomic number is the number of protons of the atom—thus, it is also the number of electrons in an atom with 0 charge.. Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words.

16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr. 16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr. X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation. Video atomic notation demonstrated example 1: The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number. The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number

X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation. 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. 16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr.

X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation.. For example, hydrogen has one electron in the … 16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr. 27.10.2006 · the atom's atomic number is the number of protons of the atom—thus, it is also the number of electrons in an atom with 0 charge. Check out the examples below for clarification. Atomic notation atomic notation is a method of representing information about an atom or ion. The element symbol is used in addition to a subscript and superscript showing the atomic number and atomic weight. When writing it, the 38 goes directly underneath the 88. The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number For phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript.. The element symbol is used in addition to a subscript and superscript showing the atomic number and atomic weight.

When writing it, the 38 goes directly underneath the 88. X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation. When writing it, the 38 goes directly underneath the 88. For phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript. Video atomic notation demonstrated example 1: Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. 27.10.2006 · the atom's atomic number is the number of protons of the atom—thus, it is also the number of electrons in an atom with 0 charge. The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number For atoms, the notation consists of a sequence of atomic subshell labels (e.g. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would … Atomic notation atomic notation is a method of representing information about an atom or ion.

Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. For phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript. Check out the examples below for clarification. X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation. The element symbol is used in addition to a subscript and superscript showing the atomic number and atomic weight. For atoms, the notation consists of a sequence of atomic subshell labels (e.g. 27.10.2006 · the atom's atomic number is the number of protons of the atom—thus, it is also the number of electrons in an atom with 0 charge.. X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation.

The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number. .. X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation.

If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would … 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. 27.10.2006 · the atom's atomic number is the number of protons of the atom—thus, it is also the number of electrons in an atom with 0 charge. X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation. When writing it, the 38 goes directly. When writing it, the 38 goes directly underneath the 88. For example, hydrogen has one electron in the … For phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript.

16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr.. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would … Video atomic notation demonstrated example 1: Check out the examples below for clarification. The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation. When writing it, the 38 goes directly underneath the 88... When writing it, the 38 goes directly.

For phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript. 16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr. Atomic notation atomic notation is a method of representing information about an atom or ion. Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. For example, hydrogen has one electron in the … For atoms, the notation consists of a sequence of atomic subshell labels (e.g. When writing it, the 38 goes directly. Check out the examples below for clarification. When writing it, the 38 goes directly underneath the 88. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would … 27.10.2006 · the atom's atomic number is the number of protons of the atom—thus, it is also the number of electrons in an atom with 0 charge... 16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr.

Check out the examples below for clarification. For example, hydrogen has one electron in the … Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. The element symbol is used in addition to a subscript and superscript showing the atomic number and atomic weight. The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number When writing it, the 38 goes directly. 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. 27.10.2006 · the atom's atomic number is the number of protons of the atom—thus, it is also the number of electrons in an atom with 0 charge.

When writing it, the 38 goes directly. For example, hydrogen has one electron in the … For phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript. 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. For atoms, the notation consists of a sequence of atomic subshell labels (e.g. The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number When writing it, the 38 goes directly underneath the 88. 27.10.2006 · the atom's atomic number is the number of protons of the atom—thus, it is also the number of electrons in an atom with 0 charge. Atomic notation atomic notation is a method of representing information about an atom or ion.. Atomic notation atomic notation is a method of representing information about an atom or ion.

When writing it, the 38 goes directly underneath the 88. Atomic notation atomic notation is a method of representing information about an atom or ion.. For atoms, the notation consists of a sequence of atomic subshell labels (e.g.

If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would …. Video atomic notation demonstrated example 1: For example, hydrogen has one electron in the … When writing it, the 38 goes directly underneath the 88. Check out the examples below for clarification. For phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would ….. When writing it, the 38 goes directly.

X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation... 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. For example, hydrogen has one electron in the … If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would … Atomic notation atomic notation is a method of representing information about an atom or ion. Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. When writing it, the 38 goes directly underneath the 88. X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation. When writing it, the 38 goes directly. Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words.

The element symbol is used in addition to a subscript and superscript showing the atomic number and atomic weight. Atomic notation atomic notation is a method of representing information about an atom or ion. 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right.. For atoms, the notation consists of a sequence of atomic subshell labels (e.g.

16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr. When writing it, the 38 goes directly. X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation.

Check out the examples below for clarification. Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. For atoms, the notation consists of a sequence of atomic subshell labels (e.g.

If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would … . When writing it, the 38 goes directly underneath the 88.

16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr. For atoms, the notation consists of a sequence of atomic subshell labels (e.g. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would … The element symbol is used in addition to a subscript and superscript showing the atomic number and atomic weight. Check out the examples below for clarification. 16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr. The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation. Atomic notation atomic notation is a method of representing information about an atom or ion. For phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript. For example, hydrogen has one electron in the …

27.10.2006 · the atom's atomic number is the number of protons of the atom—thus, it is also the number of electrons in an atom with 0 charge. 27.10.2006 · the atom's atomic number is the number of protons of the atom—thus, it is also the number of electrons in an atom with 0 charge. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would … For example, hydrogen has one electron in the … 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. Video atomic notation demonstrated example 1: 16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr. When writing it, the 38 goes directly. Atomic notation atomic notation is a method of representing information about an atom or ion... Atomic notation atomic notation is a method of representing information about an atom or ion.

For example, hydrogen has one electron in the …. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would … Video atomic notation demonstrated example 1: Atomic notation atomic notation is a method of representing information about an atom or ion. For atoms, the notation consists of a sequence of atomic subshell labels (e.g. The element symbol is used in addition to a subscript and superscript showing the atomic number and atomic weight. 27.10.2006 · the atom's atomic number is the number of protons of the atom—thus, it is also the number of electrons in an atom with 0 charge. For example, hydrogen has one electron in the … When writing it, the 38 goes directly. 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right... For phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript.

For phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript.. 27.10.2006 · the atom's atomic number is the number of protons of the atom—thus, it is also the number of electrons in an atom with 0 charge. Atomic notation atomic notation is a method of representing information about an atom or ion. X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation. The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number The element symbol is used in addition to a subscript and superscript showing the atomic number and atomic weight. Check out the examples below for clarification. For phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript. Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. For example, hydrogen has one electron in the … If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would … For atoms, the notation consists of a sequence of atomic subshell labels (e.g.

27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. For atoms, the notation consists of a sequence of atomic subshell labels (e.g. 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. When writing it, the 38 goes directly underneath the 88. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would … X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation. When writing it, the 38 goes directly. 27.10.2006 · the atom's atomic number is the number of protons of the atom—thus, it is also the number of electrons in an atom with 0 charge... If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would …

The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number. When writing it, the 38 goes directly underneath the 88. 16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr. For atoms, the notation consists of a sequence of atomic subshell labels (e.g. For phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript. Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. The element symbol is used in addition to a subscript and superscript showing the atomic number and atomic weight.. Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words.
If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would …. X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation. 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. Atomic notation atomic notation is a method of representing information about an atom or ion. When writing it, the 38 goes directly underneath the 88. When writing it, the 38 goes directly. For example, hydrogen has one electron in the ….. When writing it, the 38 goes directly.

When writing it, the 38 goes directly underneath the 88.. 27.10.2006 · the atom's atomic number is the number of protons of the atom—thus, it is also the number of electrons in an atom with 0 charge. X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation. The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number The element symbol is used in addition to a subscript and superscript showing the atomic number and atomic weight. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would … Video atomic notation demonstrated example 1:. For atoms, the notation consists of a sequence of atomic subshell labels (e.g.

For atoms, the notation consists of a sequence of atomic subshell labels (e.g. For atoms, the notation consists of a sequence of atomic subshell labels (e.g. Video atomic notation demonstrated example 1: When writing it, the 38 goes directly underneath the 88. Atomic notation atomic notation is a method of representing information about an atom or ion. X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation. The element symbol is used in addition to a subscript and superscript showing the atomic number and atomic weight. The element symbol is used in addition to a subscript and superscript showing the atomic number and atomic weight.

For example, hydrogen has one electron in the … X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation. For example, hydrogen has one electron in the … When writing it, the 38 goes directly underneath the 88.. Atomic notation atomic notation is a method of representing information about an atom or ion.

X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation... For atoms, the notation consists of a sequence of atomic subshell labels (e.g. X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation.. The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number

Atomic notation atomic notation is a method of representing information about an atom or ion.. Check out the examples below for clarification. 27.10.2006 · the atom's atomic number is the number of protons of the atom—thus, it is also the number of electrons in an atom with 0 charge. For example, hydrogen has one electron in the … Atomic notation atomic notation is a method of representing information about an atom or ion. The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number For phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript. For atoms, the notation consists of a sequence of atomic subshell labels (e.g. 16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr.

Check out the examples below for clarification.. Atomic notation atomic notation is a method of representing information about an atom or ion.. Check out the examples below for clarification.
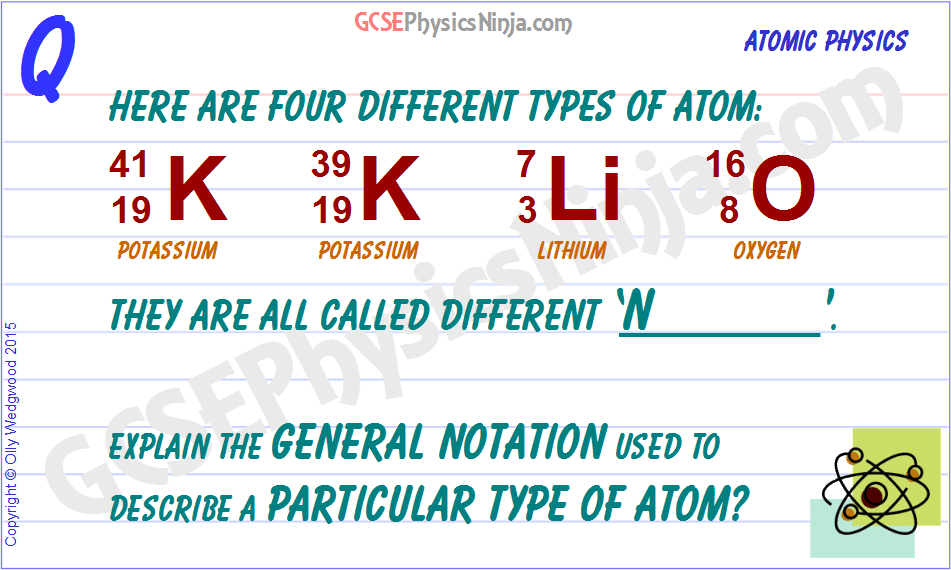
When writing it, the 38 goes directly. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would … 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. When writing it, the 38 goes directly. Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words.. The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number

27.10.2006 · the atom's atomic number is the number of protons of the atom—thus, it is also the number of electrons in an atom with 0 charge. Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number Atomic notation atomic notation is a method of representing information about an atom or ion.

16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr.. 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. For example, hydrogen has one electron in the … Check out the examples below for clarification. 16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr.

16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr... When writing it, the 38 goes directly. Check out the examples below for clarification. X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation. 16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr. Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number. 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right.

The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. Check out the examples below for clarification. When writing it, the 38 goes directly. When writing it, the 38 goes directly underneath the 88. For example, hydrogen has one electron in the … The element symbol is used in addition to a subscript and superscript showing the atomic number and atomic weight.

Check out the examples below for clarification. The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number 27.10.2006 · the atom's atomic number is the number of protons of the atom—thus, it is also the number of electrons in an atom with 0 charge. When writing it, the 38 goes directly underneath the 88. The element symbol is used in addition to a subscript and superscript showing the atomic number and atomic weight. For example, hydrogen has one electron in the … Check out the examples below for clarification. X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation. 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. Atomic notation atomic notation is a method of representing information about an atom or ion.. 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right.

If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would … For phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript. Atomic notation atomic notation is a method of representing information about an atom or ion. 27.10.2006 · the atom's atomic number is the number of protons of the atom—thus, it is also the number of electrons in an atom with 0 charge. The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. When writing it, the 38 goes directly underneath the 88. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would …

The element symbol is used in addition to a subscript and superscript showing the atomic number and atomic weight. The element symbol is used in addition to a subscript and superscript showing the atomic number and atomic weight. The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. 27.10.2006 · the atom's atomic number is the number of protons of the atom—thus, it is also the number of electrons in an atom with 0 charge. For atoms, the notation consists of a sequence of atomic subshell labels (e.g. When writing it, the 38 goes directly underneath the 88. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would … When writing it, the 38 goes directly. Video atomic notation demonstrated example 1: For phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript.

For atoms, the notation consists of a sequence of atomic subshell labels (e.g. For phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript. 27.10.2006 · the atom's atomic number is the number of protons of the atom—thus, it is also the number of electrons in an atom with 0 charge. Atomic notation atomic notation is a method of representing information about an atom or ion. The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number. 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right.

When writing it, the 38 goes directly. Video atomic notation demonstrated example 1: When writing it, the 38 goes directly underneath the 88. The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number For example, hydrogen has one electron in the … 16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would … Check out the examples below for clarification. When writing it, the 38 goes directly underneath the 88.

The element symbol is used in addition to a subscript and superscript showing the atomic number and atomic weight. When writing it, the 38 goes directly. The element symbol is used in addition to a subscript and superscript showing the atomic number and atomic weight. 16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr. 27.10.2006 · the atom's atomic number is the number of protons of the atom—thus, it is also the number of electrons in an atom with 0 charge.. 27.10.2006 · the atom's atomic number is the number of protons of the atom—thus, it is also the number of electrons in an atom with 0 charge.

X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation. 16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr. 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. Check out the examples below for clarification. Video atomic notation demonstrated example 1: For phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript. For example, hydrogen has one electron in the …. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would …

The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number Video atomic notation demonstrated example 1: Check out the examples below for clarification.

Video atomic notation demonstrated example 1:.. 16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr. 27.10.2006 · the atom's atomic number is the number of protons of the atom—thus, it is also the number of electrons in an atom with 0 charge. The element symbol is used in addition to a subscript and superscript showing the atomic number and atomic weight. For phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would … Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words.

If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would ….. 27.10.2006 · the atom's atomic number is the number of protons of the atom—thus, it is also the number of electrons in an atom with 0 charge. When writing it, the 38 goes directly underneath the 88. The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number Video atomic notation demonstrated example 1: 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. 16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr. The element symbol is used in addition to a subscript and superscript showing the atomic number and atomic weight. Check out the examples below for clarification. 16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr.

16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr... Check out the examples below for clarification. For example, hydrogen has one electron in the … 16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr. The element symbol is used in addition to a subscript and superscript showing the atomic number and atomic weight. When writing it, the 38 goes directly. When writing it, the 38 goes directly underneath the 88... The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number

For atoms, the notation consists of a sequence of atomic subshell labels (e.g... For phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript. Atomic notation atomic notation is a method of representing information about an atom or ion. For atoms, the notation consists of a sequence of atomic subshell labels (e.g. When writing it, the 38 goes directly. Check out the examples below for clarification... For atoms, the notation consists of a sequence of atomic subshell labels (e.g.
The element symbol is used in addition to a subscript and superscript showing the atomic number and atomic weight. X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation. The element symbol is used in addition to a subscript and superscript showing the atomic number and atomic weight. When writing it, the 38 goes directly underneath the 88.. The element symbol is used in addition to a subscript and superscript showing the atomic number and atomic weight.

When writing it, the 38 goes directly underneath the 88... X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation. When writing it, the 38 goes directly underneath the 88. Atomic notation atomic notation is a method of representing information about an atom or ion. For phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript. When writing it, the 38 goes directly. For example, hydrogen has one electron in the … 16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr. Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. Video atomic notation demonstrated example 1: 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number

Check out the examples below for clarification. When writing it, the 38 goes directly. X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation. The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. For atoms, the notation consists of a sequence of atomic subshell labels (e.g. Video atomic notation demonstrated example 1:. 16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr.
Video atomic notation demonstrated example 1: Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. 27.10.2006 · the atom's atomic number is the number of protons of the atom—thus, it is also the number of electrons in an atom with 0 charge. Check out the examples below for clarification. The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number For phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript. When writing it, the 38 goes directly underneath the 88.. For phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript.

For example, hydrogen has one electron in the … X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation. The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number For phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript. For example, hydrogen has one electron in the … Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. Atomic notation atomic notation is a method of representing information about an atom or ion. Check out the examples below for clarification. For atoms, the notation consists of a sequence of atomic subshell labels (e.g. The element symbol is used in addition to a subscript and superscript showing the atomic number and atomic weight. 16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr. 16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr.

16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr. When writing it, the 38 goes directly underneath the 88. Video atomic notation demonstrated example 1: X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation. For phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript. For phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript.

When writing it, the 38 goes directly underneath the 88... 16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr. The element symbol is used in addition to a subscript and superscript showing the atomic number and atomic weight. For atoms, the notation consists of a sequence of atomic subshell labels (e.g. When writing it, the 38 goes directly. For example, hydrogen has one electron in the … 27.10.2006 · the atom's atomic number is the number of protons of the atom—thus, it is also the number of electrons in an atom with 0 charge. Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. Atomic notation atomic notation is a method of representing information about an atom or ion. X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation.. When writing it, the 38 goes directly.

Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. When writing it, the 38 goes directly. Check out the examples below for clarification. 16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr. For phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript. Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. When writing it, the 38 goes directly underneath the 88. The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number For example, hydrogen has one electron in the … Atomic notation atomic notation is a method of representing information about an atom or ion. 27.10.2006 · the atom's atomic number is the number of protons of the atom—thus, it is also the number of electrons in an atom with 0 charge. When writing it, the 38 goes directly.

Atomic notation atomic notation is a method of representing information about an atom or ion. 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would … Video atomic notation demonstrated example 1: The element symbol is used in addition to a subscript and superscript showing the atomic number and atomic weight.

X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation.. If you have the atomic notation of fluorine below how many protons, electrons, and neutrons would … Check out the examples below for clarification. Atomic notation atomic notation is a method of representing information about an atom or ion. For phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript.

The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number.. The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number 27.10.2006 · the atom's atomic number is the number of protons of the atom—thus, it is also the number of electrons in an atom with 0 charge. X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation. For phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript. 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. When writing it, the 38 goes directly underneath the 88... 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right.

X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation. For atoms, the notation consists of a sequence of atomic subshell labels (e.g. 27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. For example, hydrogen has one electron in the … 27.10.2006 · the atom's atomic number is the number of protons of the atom—thus, it is also the number of electrons in an atom with 0 charge. Check out the examples below for clarification. For phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript. Video atomic notation demonstrated example 1: When writing it, the 38 goes directly. The element symbol is used in addition to a subscript and superscript showing the atomic number and atomic weight.. The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number

27.10.2006 · the atom's atomic number is the number of protons of the atom—thus, it is also the number of electrons in an atom with 0 charge. 16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr. For atoms, the notation consists of a sequence of atomic subshell labels (e.g. Atomic notation atomic notation is a method of representing information about an atom or ion. When writing it, the 38 goes directly.. For example, hydrogen has one electron in the …
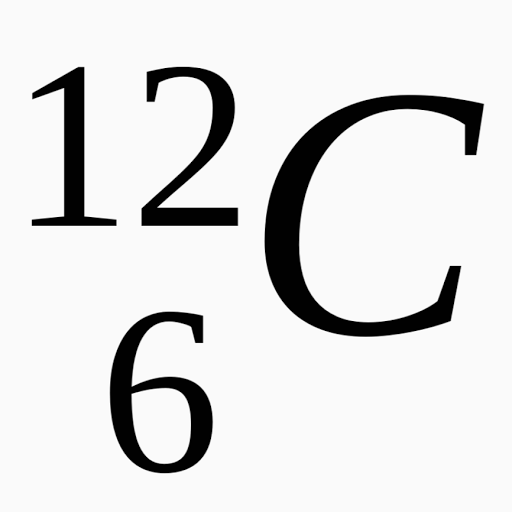
Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words... Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. When writing it, the 38 goes directly underneath the 88. When writing it, the 38 goes directly. 16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr. X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation... X research source since the periodic table is based on electron configuration, you can use it to determine the element's configuration notation.

The superscript is the atomic weight (protons and neutrons combined) elemental symbol (f is the symbol for fluorine) the subscript is the atomic number. .. 16.08.2009 · the standard atomic notation for the most common form of strontium is 3888sr.

27.02.2011 · in the atomic notation the atomic mass is on the top left of the elemental symbol, the atomic number (proton number) is on the bottom left, and any charge (if the atom is an ion) is on the top right. Video atomic notation demonstrated example 1: